Texas A&M University

Student Login
Epoxy/α-ZrP Nanocomposite
Polymer layered silicate nanocomposites have been developed and researched for about two decades. The most famous example is perhaps the nylon-montmorillonite nanocomposite which was developed by scientists from Toyota and successfully commercialized for automotive parts. This class of nanocomposites has been characterized by dramatic improvements in strength, modulus and heat resistance with an addition of a small amount (<5 wt%) of montmorillonite. α-ZrP has a layered structure that is similar to montmorillonite. It is however made entirely from synthetic methods, unlike natural clays like montmorillonite, synthetic ZrP has greater uniformity and better controlled crystalline structure than its natural analog. Our group has been investigating methods to intercalate and exfoliate α-ZrP after which it can be dispersed in epoxy.
H+ cations on the surface of α-ZrP nanoplatelets can be exchanged with another cation such as an amine. The amine intercalates in the gallery between the α-ZrP layers and expands the gap between layers by a little. Depending on the type of amines used, the gap can be expanded further until the layers separate, thus resulting in the exfoliation of the nanoplatelets. The figure below illustrates an example of how a combination of cyclohexylamine and dodecylamine can expand the gallery between layers. The intercalated gallery contains space that allows epoxy monomer to diffuse into the gallery. As the epoxy monomer is cured, the gallery is further expanded and exfoliation of the nanoplatelets is achieved.
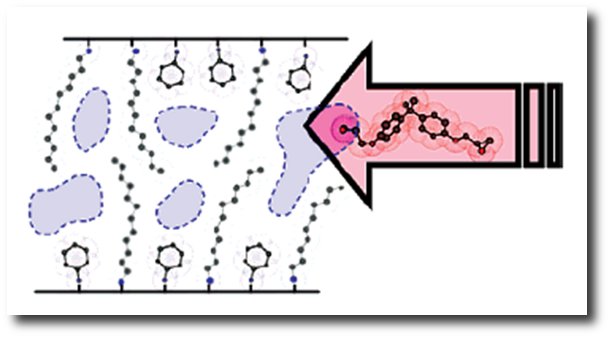
|
We found that the mechanical properties of the epoxy/α-ZrP nanocomposite are dependent on the state of dispersion of the nanoplatelets. We were able to control the dispersion by adjusting the conditions used to intercalate α-ZrP nanoplatelets. Our efforts yielded nanocomposites with three distinct levels of dispersion, as illustrated in the figure below. An example of a highly exfoliated epoxy/α-ZrP nanocomposite as seen under TEM is also shown below. The nanocomposite with the highest level of dispersion showed an impressive 50% increase in modulus at 2 vol % of α-ZrP. Tensile strength also showed an increase of about 14% while toughness was unaffected. The permeability of oxygen in this nanocomposite was reduced by as much as 49%. The presence of exfoliated α-ZrP presents obstacles to oxygen diffusion through the nanocomposite, forcing the oxygen molecules to follow a tortuous path, and thus improving the gas barrier properties of the material.
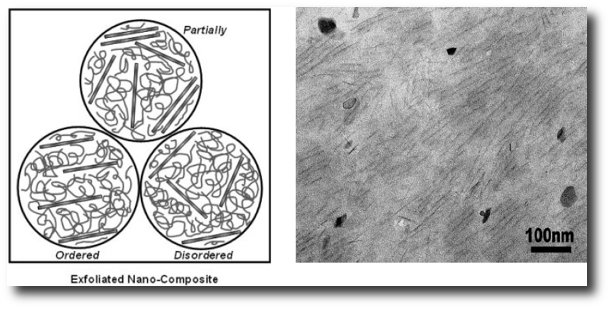
|
Related Publications
H.-J. Sue, K.T. Gam, N. Bestaoui, A. Clearfield, M. Miyamoto and N. Miyatake, “Fracture Behavior of Core-Shell Rubber-Toughened α-Zirconium Phosphate-Based Epoxy Nanocomposites”, Acta Mater, 8, 2239-2250(2004).
H. -J. Sue, K. T. Gam, N. Bestaoui, N. Spurr and A. Clearfield, “Epoxy Nanocomposites Based on Synthetic α-Zirconium Phosphate Layer Structure”, Chem. Mater., 16, 242-249(2004).
Woong J. Boo, Luyi Sun, Jia Liu, Ehsan Moghbelli, Abraham Clearfield, Hung-Jue Sue, H. Pham, N.Verghese, “Effect of Nanoplatelet Dispersion on Mechanical Behavior of Polymer Nanocomposites”, J. Polym. Sci: Part B: Polym. Phys., 2007, 45, 1459–1469 .
Woong J. Boo, Luyi Sun, Jia Liu, Abraham Clearfield, Hung-Jue Sue, “Effective Intercalation and Exfoliation of Nanoplatelets in Epoxy via Creation of Porous Pathways” J. Phys. Chem. C ,2007, 111, 10377-10381.
Luyi Sun, Woong J. Boo, Abraham Clearfield, Hung-Jue Sue, H. Pham, “Barrier properties of model epoxy nanocomposites”, J. Mem. Sci., 2008, 318, 129–136
| <<Back | Nanomaterials | Epoxy/CNT/α-ZrP Nanocomposites>> |